Perspectives of Aquatic Toxicology/Aquatic Toxicity Tests
Chapter One: Aquatic Toxicity Tests
[edit | edit source]INTRODUCTION
[edit | edit source]Aquatic species are vital to our planet. Phytoplankton, algal plankton, and kelp are major sources of the planet’s oxygen. They absorb and store carbon dioxide, and maintain a hospitable climate. They also play an important role in the global nitrogen cycle and support aquatic animals such as fish, mollusks, sponges, and corals. Aquatic species help maintain the earth’s ecosystem and help preserve its rich biodiversity as well as providing food, medicine, livelihoods, tourism, and recreational opportunities1.
It is therefore essential to protect the planet’s rich and diverse aquatic life, and combat the many threats facing aquatic organisms including climate change, habitat destruction, overfishing, the introduction of invasive species, and chemical pollution2. This chapter will focus on chemical pollution. The risks to aquatic life can be minimized and better managed by understanding how chemicals impact it.
There are more than 140,000 man-made chemicals in the environment3, with the United States alone producing 2000 new chemicals every year4. It is conceivable that aquatic species are exposed to many of these chemicals on an acute (short-term) and chronic (long-term) basis, although there is an absence of data to indicate how many of these chemicals are released into various water bodies. Chemical exposure can affect organisms’ growth, development, fecundity, behavior, and survival, among other biological processes. Hence, it is important to test chemical toxicity before it is released into the environment in order to determine maximum acceptable toxicant concentrations (see section II of this chapter) and to protect species from potential harm.
Toxicity testing is done to identify the degree to which chemicals can damage living organisms in a controlled environment. It has four major objectives:
a. To obtain toxicity and exposure data for various chemicals
b. To aid in estimating and managing risks posed by various chemicals
c. To aid in setting chemical regulations and environmental standards
d. To classify chemicals based on how toxic they are to various species
The dose makes the poison in toxicology. It is possible to determine safe and unsafe doses, or concentrations, for nearly every chemical. For example, the most toxic substance on earth, the bacteria-produced botulinum toxin, can kill humans with a very small dose, but it can be used safely in Botox5.
Risk is a function of toxicity and exposure. A chemical can be very toxic, but it will have zero risk to aquatic organisms if it never enters water bodies (i.e., there is no exposure). The maximum allowable concentration for a chemical in the environment is based on the risk it poses to various species. An acceptable “safe” concentration is usually one that does not harm 95% of the species.
Many questions can be answered by carrying out toxicity tests:
a. At what concentration is a chemical non-toxic to an organism? At what concentration is it toxic?
b. What effects can be observed from short-term and long-term chemical exposure?
c. Which chemicals are the most and least toxic to an organism?
d. Which organisms are the most or least sensitive to a chemical?
e. Are some life stages of an organism more sensitive?
f. Do certain environmental conditions make a chemical more toxic?
g. Is the toxicity of a chemical similar in lab and in the outside environment?
h. What is the effect of a mixture of chemicals?
And more.
Aquatic organisms can be exposed to chemicals when effluents and sewage are released into water bodies. Sometimes chemicals inadvertently enter water through oil spill or runoffs from agricultural fields. Chemicals present in the air can be deposited into water bodies either directly (dry deposition) or through rainfall, snowfall, and fog (wet deposition). Some of the chemicals commonly found in water bodies include detergents, fertilizers, pesticides, pharmaceuticals, food and cosmetic preservatives, chemicals used in kitchenware and plastic, and metals6-8. Aquatic animals such as fish can take up these chemicals via their gills, absorb them through their integument, and/or ingest them. Aquatic plants that have vascular systems can absorb chemicals through their epidermal surface and/or roots. Plants that are not completely submerged in water can take up chemicals in the air through their stomata.
Chemical properties and type of aquatic species determine how chemicals are taken up, distributed, stored, metabolized, and excreted. Hydrophobic (fat-loving) chemicals are more likely to enter a fish’s body, and warm temperatures typically increase the uptake as the fat become more fluid-like. Smaller, uncharged molecules also cross membranes more easily. Hydrophilic (water-loving) chemicals are more likely to be transported by the circulatory system. On the other hand, hydrophobic chemicals are more likely to bind to molecules and accumulate in fat bodies. While chemical storage is protective in the short term (they are not free to move and act), they can be released later and cause toxicity. This usually happens when an organism breaks down fat for greater energy needs, i.e., during illness, starvation, or reproduction.
A species’ metabolic enzymes often modify a chemical in order to detoxify its effects, but this modification can sometimes make a chemical more toxic. Chemicals with many halogen atoms such as chlorine, and fluorine are often difficult to modify. Many aquatic animals eliminate chemicals through their gills or skin. Further details on chemical biotransformation can be found in the Biotransformation of Xenobiotics Chapter of this book.
Chemical exposure can kill or harm aquatic organisms directly through such means as growth reduction, delayed development, decreased fertility, and behavioral changes, or can reduce or eliminate its food supply by killing its prey, or limiting its shelter through habitat destruction. This can lead to increased competition for food and shelter, disrupting the food web, and altering the ecological balance.
BASIC CONCEPTS
[edit | edit source]Units of concentration
[edit | edit source]Concentration is used more commonly than dose in aquatic toxicology. This is because water chemical concentration is easier to measure than the amount of chemical taken up by fish through its gills, integument, and mouth. Concentration of a solution is defined as the ratio of solute to solvent, or the ratio of solute to total solution. This can be either expressed as mass of chemical per unit volume (e.g. mg/mL) or the number of moles of chemicals per liter of solution (e.g. mol/L).
Terms like ppm, ppb and ppt are also often used to describe units of concentration:
a. Parts per million (ppm) corresponds to 1 mg of chemical/L of solution. The amount of chemical is six orders of magnitude lesser than the amount of solution. This is like emptying a large soft drink bottle of a chemical into an Olympic-sized swimming pool.
b. Parts per billion (ppb) corresponds to 1 µg of chemical/L of solution. The amount of chemical is nine orders of magnitude lesser than the amount of solution. This is like putting half a teaspoon of a chemical into an Olympic-sized swimming pool.
c. Parts per trillion (ppm) corresponds to 1 ng of chemical/L of solution. The amount of chemical is twelve orders of magnitude lesser than the amount of solution. This is like putting one-twentieth of a drop of chemical into an Olympic-sized swimming pool.
Concentration and response
[edit | edit source]There is a relationship between the chemical concentration to which an organism has been exposed and the resultant nature and degree of harmful effects. However, it is important to note that the chemical concentration that enters an organism is typically higher than the concentration that causes a toxic effect. This could be due to the organism producing enzymes or molecules that break down or bind to the chemical. This reduces the availability of the chemical to the body, and thus a lower concentration binds to the site of action and exerts a toxic effect.
Assumptions in a concentration-response relationship are as follows:
a. It is a cause-and-effect relationship, i.e., the response occurs due to the organism’s exposure to a chemical.
b. The response is due to a chemical interacting at the site of action.
c. The concentration of a chemical at the site of action is a function of how much chemical the organism was exposed to.
d. Above the threshold concentration (concentration at which a response can be detected), the magnitude of response is proportional to the amount of chemical interacting at the site of action.
e. The response can be measured and reproduced under similar conditions.
The duration of an organism’s chemical exposure is also significant. If a fish is exposed to a low chemical concentration for only one day, it may be unaffected, but if it is exposed to the same concentration for months it may develop cancer, skeletal abnormalities, issues with fertility, etc.
The response to a chemical for any given species usually follows a normal distribution or bell-shaped curve (see Figure 1). This means that some organisms of the same species are very sensitive to a given chemical, some are very resistant, while most are neither very sensitive nor very resistant.
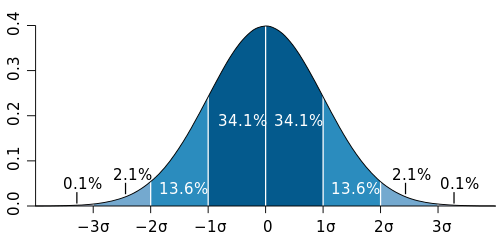
Creating a toxicity curve
[edit | edit source]A toxicity curve or a concentration-response curve is a graph which plots the results obtained from a traditional concentration-response toxicity test. The toxicity test should ideally fulfill the following criteria:
a. There should be five different concentrations of a chemical plus a negative control.
b. The concentrations should be equally spaced.
c. The concentrations should cause a range of effects, from 0% effect to 100% effect.
d. The study should be replicated at least three times.
The toxicity curve generated from such an experimental design should have the following features in most cases (see Figure 2):
a. The x-axis should be the concentration and the y-axis should be the response.
b. The curve should be sigmoidal in shape above the threshold dose.
c. The average cumulative response from the three replicated studies should be plotted with the 95% confidence intervals.
The slope of a generated toxicity curve can indicate several things. A very steep slope indicates that small increases in concentration caused large increases in response; a flat slope indicates that large increases in concentrations caused small increases in response. The curve can also be used to estimate a concentration that causes a particular response (for example, a 10% response). Typically, the concentration which causes a 50% response is calculated, as it has the least variability/noise.

A non-standard toxicity curve is observed for essential chemicals such as water, oxygen, and vitamins. There is an optimal range for these chemicals: a very low concentration causes deficiency and death, and a very high concentration causes toxicity and death (see Figure 3). Another kind of non-standard toxicity curve is seen with chemicals found in such materials as plastics. These chemicals have different modes of action at different concentrations: they can disrupt hormones at low concentrations but not at high concentrations9. Some toxicity curves can be bimodal if males and females have different toxicity thresholds to a chemical.

Measures of toxicity
[edit | edit source]Toxicity is commonly measured in toxicological studies as follows:
Lethal Concentration 50 (LC50): Concentration at which 50% of the organisms in a population are killed following chemical exposure.
Effective Concentration 50 (EC50): Concentration at which 50% of the organisms in a population are affected following chemical exposure. The effects observed could be reduced growth, delayed development, etc.
Inhibitory Concentration 50 (IC50): Concentration at which 50% of organisms in a population are inhibited following chemical exposure. The chemical could have inhibited a specific biological or biochemical function.
Often LC/EC/IC 10 (harm to 10% of population) and LC/EC/IC 90 (harm to 90% of population) are also calculated.
No Observed Effect Concentration (NOEC): Highest chemical concentration that does not cause a toxic effect in the treated population.
Lowest Observed Effect Concentration (LOEC): Lowest chemical concentration that causes a toxic effect in the treated population.
Maximum Acceptable Toxicant Concentration (MATCC): Concentration between NOEC and LOEC, or is a geometric mean of the two. It is calculated for chronic studies only.
TOXIC EFFECTS OF CHEMICALS
[edit | edit source]Chemicals can exert toxic effects on organisms through various mechanisms:
a. Binding: Chemicals can bind to molecules on the surface of cells and disrupt communication between cells. If an organism’s cells do not communicate with each other they will not function normally. Chemicals can also bind to enzymes and prevent them from carrying out essential activities such as digestion and metabolism, as well as bind to DNA and change the amount of proteins produced. A correct number of proteins are needed to build cells, produce hormones, and maintain immunity.
b. Bioaccumulation: This occurs when an organism takes up a chemical faster than it eliminates it. In bioaccumulation the chemical is taken up by contact, respiration, and ingestion. The term bioconcentration is used when the chemical is taken up through contact and respiration only. A chemical accumulation in living tissue can poison tissue, and subsequently, organs.
c. Interaction: Two or more chemicals in an organism can interact with one another. Usually, the combined chemical effect will equal the sum of their individual effects (1 + 2 = 3). This is called an additive effect and can be observed when aspirin and acetaminophen are taken together. They both act in a similar manner and their combined effect is comparable to taking two doses of one drug. Another kind of interaction occurs when the combined effect of two chemicals are greater than the sum of their individual effects (1 + 2 = 5), and can happen if chemical A increases the activity of chemical B. This is called the synergistic or potentiation effect and can be observed when acetaminophen and alcohol are taken together. Both are broken down by the liver and their combined presence taxes the organ, making it more vulnerable to failure. The final kind of interaction occurs when the combined effect of two chemicals is less than the sum of their individual effects (1 + 2 = 1). This occurs if chemical A hinders the activity of chemical B, and is called an antagonistic effect. A classic example of this effect is anti-venom drugs canceling the effects of a snake bite.
The intracellular effects of a chemical can also be described with the help of a toxicity pathway. It is a sequence of events, starting with a chemical entering an organism. A proportion of the chemical reaches the target tissue and interacts with it: for example, by binding to cell receptors on the tissue. This causes a perturbation, or disturbance, in normal cell function. If the cell starts to alter and the organism corrects the change in time, there will not be a problem; however, if the organism does not alter and effect change, the cell will be permanently altered/injured10 (see Figure 4).

Adverse Outcome Pathways (AOPs) encompass toxicity pathways and beyond. That is, they include chemical-molecular interactions and cellular changes, and determine how this leads to changes in organs, organisms, and populations. In Figure 5 an AOP has been drawn for a male fish exposed to a chemical that activates the estrogen receptor. At the cellular stage this could lead to the transcription and production of abnormal proteins. These proteins could cause both ovaries and testis to develop, altering the secondary sex characteristics of the fish and impairing its fertility. The sex ratio could become skewed if many males in a population become feminized and infertile11.

Table 1 shows how toxicity endpoints could manifest in various aquatic organisms. The length of chemical exposure (short-term vs. long-term) often determines which toxicity endpoints should be measured. For example, reproductive problems and tumors are only observed with long-term exposures.
Table 1: Toxicity endpoints for different aquatic species following exposure to chemicals
| SPECIES | TOXICITY ENDPOINTS COMMONLY MEASURED |
| Annelids | Growth, fecundity, bioaccumulation |
| Algae | Growth, biomass, coloration |
| Plants | Growth, length, yield |
| Insects | Mortality, immobility, development, fecundity, emergence, sex ratio |
| Mollusks | Mortality, growth, fecundity, bioconcentration |
| Crustaceans | Mortality, growth, immobility, fecundity |
| Amphibians | Mortality, growth, development, length, histopathology, metamorphosis, reproductive maturity |
| Fish | Mortality, no heartbeat, loss of swimming equilibrium, developmental deformities, length, yolk coagulation, growth, skeletal abnormalities, tumors, reproductive maturation, fecundity, histopathology, egg hatching, behavior, etc. |
TYPES OF TOXICITY TESTS
[edit | edit source]Aquatic toxicity tests can be divided into categories as described below and summarized in Figure 6.

Based on duration of chemical exposure
[edit | edit source]a. Acute: Short-term tests. For fish the tests are 24-96h long; but, for microalgae or bacteria, a 96h test could represent a chronic, life cycle, or multigenerational test. The test is commonly carried out to check chemical lethality.
b. Subchronic: Prolonged acute tests. For fish the tests are typically anywhere between 28 days to 3 months long. The test is usually done to determine if a chemical impacts the growth (body mass) of a species.
c. Chronic: Lasts for at least 10% of the tested species’ lifespan. For invertebrates, 21-day chronic studies are common. The test typically lasts longer than 6 months for fish. It is often conducted to see if a chemical causes reproductive and developmental effects.
d. Life cycle: Lasts through an organism’s entire life cycle. This can be from egg to sexual maturity, or from egg to egg. This test is done to check if a chemical causes developmental or reproductive effects, as with chronic studies.
e. Multigenerational: Carried out on two or more consecutive generations (parents and offspring). It is usually performed to examine if offspring are affected by parental exposure to chemicals.
f. Early-life-stage: Done on embryos or larval stages. Different life stages of an organism can exhibit different sensitivities to a chemical, with early-life stages often more susceptible.
Based on exposure systems
[edit | edit source]a. Static: Organisms are placed in still water containing a chemical (or in control water). The water is not changed during the test. This system is widely used, but for studies longer than 24h they may not accurately represent chemical effects. This is because the concentration of the chemical may change over time and toxic effects may be produced from a build-up of metabolic byproducts released by the organisms.
b. Renewal: Similar to static, with the test conducted in still water. However, in this test the water containing the chemical (or control water) is regularly changed during the test, usually every 24h, ensuring that the chemical concentration remains stable and that organisms are exposed to clean and fresh water daily.
c. Recirculation: Also similar to static, but the water containing the chemical (or control water) is filtered. This ensures that the water quality does not deteriorate over time. However, filters can add uncertainties to the study as the filter media may interact with the test chemical.
d. Flow-through: Water containing the chemical (or control water) constantly flows in and out of the system, maintaining a high quality flow where the influent and effluent never mix. Pumps control the flow of water and dilutors ensure that the right concentration of chemical is delivered. Flow-through systems mimic the natural flow of water, and though expensive, are regularly used for this reason.
Based on toxicity endpoints
[edit | edit source]These tests are done to determine if a chemical could cause one or more toxicity endpoints in a test organism. The endpoints frequently analyzed include mortality, growth, development, reproduction, immobilization, respiration, endocrine effects, and chemical bioaccumulation. Most standardized studies have been developed with these endpoints in mind.
Based on experimental complexity
[edit | edit source]a. Single species: Tests are conducted on a single species in a lab. They are simple and inexpensive to conduct, and constitute the most common type of test. They are often carried out in a flask, beaker, or some other glass container.
b. Microcosm: Tests conducted on two or more species in an artificial and controlled system. They represent a simplified ecosystem. Microcosms should contain less than 1000 liters of water and can be done indoors (e.g. fish tank) or outdoors (e.g. small ponds).
c. Mesocosms: Tests conducted on multiple species placed in experimental water enclosures. Mesocosms represent a complex ecosystem and mimic natural conditions. The volume of water in the system must exceed 1000 liters, and the test is usually done outdoors. More details on this testing method can be found in the Mesocosm Chapter of this book.
d. Macrocosms: Tests conducted in lakes and on whole aquatic ecosystems. They are the most realistic, but they are very difficult and expensive to conduct. Canada has 58 experimental lakes that are designated for macrocosm studies only.
Based on test media
[edit | edit source]a. Water: Water is spiked with a single chemical or a chemical mixture, and aquatic organisms are exposed to it. The vast majority of aquatic toxicity tests are done on water. The toxicity endpoints of these organisms are compared to organisms exposed to control (non-spiked) water.
b. Whole effluent: Samples of effluents are tested by exposing aquatic organisms to them. It is important to assure that as wastewater effluents are discharged into water bodies they will not harm aquatic organisms--as prescribed by the Clean Water Act. Toxicity endpoints are measured and compared to that of organisms exposed to control water.
c. Sediment: Determines if sediments contain concentrated toxic chemicals that will harm organisms. Sediments are the ultimate repository for many chemicals that enter water bodies. Benthic species such as worms, crabs, clams, and lobsters live in or on sediments. Benthic organisms are exposed to contaminated or spiked sediments in sediment toxicity tests, and their toxicity endpoints are compared to organisms that are exposed to control sediments.
It is important to understand that the five different kinds of aquatic tests mentioned above are not independent of one another; this is just one way to classify them. For example, an acute toxicity test can be done on a single species using the static exposure system. The endpoint could be mortality, and the test could be done to check for the presence of toxic contaminants in whole effluents.
Both the Organization for Economic Co-operation and Development (OECD) and the United States Environmental Protection Agency (USEPA) publish guidelines for conducting various kinds of aquatic toxicity tests. These have been summarized in Table 2, along with a link to each test guideline.
Table 2: The different aquatic toxicity test guidelines published by OECD and EPA
| TEST NUMBER AND NAME | DURATION OF
EXPOSURE |
MAJOR ENDPOINTS MEASURED | LINK TO GUIDELINE |
| Test No. 225: Sediment-Water Lumbriculus Toxicity Test Using Spiked Sediment | Subchronic | Growth, fecundity | OECD 225 |
| Test No. 315: Bioaccumulation in Sediment-dwelling Benthic Oligochaetes | Subchronic | Bioaccumulation | OECD 315 |
| Test No. 221: Lemna sp. Growth Inhibition Test | Subchronic | Growth, yield | OECD 221 |
| Test No. 239: Water-Sediment Myriophyllum Spicatum Toxicity Test | Subchronic | Growth, length, yield | OECD 239 |
| Test No. 238: Sediment-Free Myriophyllum Spicatum Toxicity Test | Subchronic | Growth, length, yield | OECD 238 |
| Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test | Chronic | Growth, biomass, coloration | OECD 201 |
| Test No. 218: Sediment-Water Chironomid Toxicity Using Spiked Sediment | Chronic | Development, emergence | OECD 218 |
| Test No. 219: Sediment-Water Chironomid Toxicity Using Spiked Water | Chronic | Development, emergence | OECD 219 |
| Test No. 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment | Life cycle | Emergence, sex ratio, fecundity, mortality, development | OECD 233 |
| Test No. 235: Chironomus sp., Acute Immobilisation Test | Acute | Immobility | OECD 235 |
| Test No. 242: Potamopyrgus antipodarum Reproduction Test | Subchronic | Mortality, fecundity | OECD 242 |
| Test No. 243: Lymnaea stagnalis Reproduction Test | Subchronic | Mortality, fecundity | OECD 243 |
| 850.1025: Oyster Acute Toxicity Test (Shell Deposition) | Acute | Growth | EPA 1025 |
| 850.1055: Bivalve Acute Toxicity Test (Embryo-Larval) | Acute | Count of embryos and larvae | EPA 1055 |
| 850.1710: Oyster Bioconcentration Factor | Subchronic | Bioconcentration | EPA 1710 |
| Test No. 202: Daphnia sp. Acute Immobilisation Test | Acute | Immobility | OECD 202 |
| Test No. 211: Daphnia magna Reproduction Test | Chronic | Fecundity | OECD 211 |
| 850.1300: Daphnid chronic toxicity test | Chronic | Mortality, growth, fecundity | EPA 1300 |
| 850.1035: Mysid Acute Toxicity Test | Acute | Mortality | EPA 1035 |
| 850.1020: Gammarid Amphipod Acute Toxicity Test | Acute | Mortality | EPA 1020 |
| 850.1045: Penaeid Acute Toxicity Test | Acute | Mortality | EPA 1045 |
| Test No. 231: Amphibian Metamorphosis Assay | Subchronic | Growth, mortality, development, length,
histopathology |
OECD 231 |
| Test No. 241: The Larval Amphibian Growth and Development Assay (LAGDA) | Early-life-stage | Development, metamorphosis, mortality, growth, reproductive maturity | OECD 241 |
| Test No. 203: Fish, Acute Toxicity Test | Acute | Mortality | OECD 203 |
| Test No. 210: Fish, Early-life Stage Toxicity Test | Early-life-stage | Growth, length, hatching, appearance & behavior, mortality | OECD 210 |
| Test No. 212: Fish, Short-term Toxicity Test on Embryo and Sac-Fry Stages | Early-life-stage | Hatching, mortality, behavior, appearance | OECD 212 |
| Test No. 215: Fish, Juvenile Growth Test | Subchronic | Behavior, appearance, growth | OECD 215 |
| Test No. 229: Fish Short Term Reproduction Assay | Subchronic | Fecundity, yolk protein, sex characteristics | OECD 229 |
| Test No. 230: 21-day Fish Assay | Subchronic | Yolk protein, secondary sex characteristics | OECD 230 |
| Test No. 234: Fish Sexual Development Test | Subchronic | Yolk protein, sex ratio | OECD 234 |
| Test No. 236: Fish Embryo Acute Toxicity (FET) Test | Acute | Mortality, yolk coagulation, heartbeat | OECD 236 |
| Test No. 240: Medaka Extended One Generation Reproduction Test (MEOGRT) | Multi-generational | Mortality, growth, development, sex, fecundity, yolk protein | OECD 240 |
| Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure | Subchronic | Bioaccumulation | OECD 305 |
DESIGNING A TOXICITY EXPERIMENT
[edit | edit source]The results of a toxicity experiment depend largely on how it was designed. A good study design can ensure that the results obtained are valid, applicable, and reproducible. Below are the major criteria used to ensure good aquatic toxicity test design, but it is important to note that not all tests can satisfy all of the outlined criteria due to the specific nature of various toxicity tests.
a. It should be widely accepted by the general scientific community.
b. It should be standardized (i.e., carried out according to defined protocols) and the results must be replicable in different laboratories.
c. It should be easy to perform and economical.
d. The test species selected must be a well-known model organism (see below).
e. The test should cover a range of concentrations, and at least some of these concentrations should be found in the environment.
f. The duration of chemical exposure must be realistic and manageable (for example, some sharks can live for hundreds of years and it is not possible to expose them to a chemical throughout their lifespan).
g. The test should be statistically sound and robust.
h. The data obtained can be used to estimate risk.
i. The test should be sensitive enough to detect and measure the toxic effects under investigation.
j. The test should be able to predict effects to species outside the lab (i.e., in the environment) and also predict potential effects to similar species.
Major standardized aquatic tests were discussed in the above section, with protocols spelled out by the OECD and USEPA. The International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM), which is now an international organization, have also published a few aquatic test protocols and can be found on their websites (https://www.iso.org/home.html and https://www.astm.org/ respectively).
The easy to administer and economical nature of standardized tests enables them to be routinely performed. This precludes macrocosm and mesocosm tests which are very complex and difficult to perform. While all the standardized toxicity tests mentioned in Table 2 were for single species, the USEPA and OECD have published guidelines for indoor microcosms (USEPA 1900) and outdoor microcosms/mesocosms (OECD draft guidance).
The appropriate selection of test species is critical in toxicity testing. While more details on this can be found in the Model Species in Aquatic Toxicology Chapter, most toxicity tests are carried out on model species which have the following characteristics: a. High sensitivity to various chemicals. b. Easily available and abundant.
c. Easy to rear and culture in the lab and allow for various types of toxicity testing.
d. High survival rate in the lab under normal conditions.
e. Extensive and available knowledge of the organism (information on their biology, physiology, genetics, and behavior).
There is no single aquatic species used in tests that can provide answers to all questions or evaluate all chemical impacts on an ecosystem. It is therefore imperative to test several species from different classes: from algae to invertebrates to fish. Also, it is important to test different life stages in a species as every stage has a different sensitivity and a unique response. The environment in which the organism lives should also be taken into consideration (freshwater/marine and warm/cold water).
Under the United States Endangered Species Act (ESA) it is necessary to ensure that chemicals released into the environment do not harm endangered or threatened species. Since the employment of endangered and threatened species in toxicity tests is discouraged, toxicity values obtained from the most sensitive test species are often used to estimate their risk to a chemical. Also, the acceptable risk for these species is often 10-fold lower than that of more abundant species12.
Toxicity tests should not be conducted without first identifying and including one or more available Estimated Environmental Concentrations (EEC) or Actual Environmental Concentration (AEC) of a chemical. EECs are the estimated concentrations of a chemical in an environment and are usually derived through computer modeling or simple predictions. These models were developed with data obtained from laboratory and environmental studies; two such models are available on the USEPA website13. The Pesticide in Water Calculator (PWC) is used to estimate pesticide concentrations in water bodies that result from pesticide applications to land. The KABAM (KOW (based) Aquatic BioAccumulation Model) is used to estimate potential bioaccumulation of hydrophobic carbon-based pesticides in freshwater aquatic food webs. AECs on the other hand, are based solely on empirical data. They can be measured by taking a water sample from a natural water body or a tissue sample from a wild aquatic organism, making it possible with the help of analytical instruments to find the concentration/dose of chemical in the samples. Various methods and instruments are required to extract and analyze different chemicals.
Estimating the risk to a species from an acute exposure to a chemical, particularly a pesticide, constitutes a Tier I study—a simple laboratory study where worse-case estimates are used to calculate the Risk Quotient (RQ). RQ is defined as the exposure concentration divided by the toxicity concentration. The toxicity concentration is the LC50 or EC50 for an acute exposure. The exposure concentration is the peak concentration of the pesticide in water bodies. If the RQ does not exceed 0.5 for aquatic animals (that is, the exposure concentration is half the toxicity concentration) and 1.0 for aquatic plants (that is, the exposure concentration does not exceed the toxicity concentration), the risk is considered acceptable12.
A Tier II study is conducted if the Tier I RQ is exceeded. Here, the species is subchronically or chronically exposed to a pesticide. The toxicity concentration is the NOEC and the exposure concentration is the average pesticide concentration over 21-60 days in water bodies. If the RQ does not exceed 1.0 (for both aquatic animals and plants), the risk is considered acceptable; a Tier III study is carried out if it is exceeded. These investigations are chronic, life cycle, or multigenerational in nature, and can be done in the laboratory or outside (i.e., microcosm studies). Tier IV are mesocosm or macrocosm studies involving multiple species. Both Tiers III and IV usually involve a comparison of the toxicity endpoints of the pesticide-exposed populations to that of the unexposed populations. The endpoints analyzed are biomass, diversity, species richness, etc. Figure 7 summarizes the risk assessment process14. Additional information can be found in the Risk Assessment Chapter.

The design of a study depends largely on the question(s) being asked. Consideration must also be given to the physiochemical properties of the chemical being tested, its mode of action, its pattern of use, the environmental conditions, and the characteristics of the test organism. The following is a brief overview:
a. Study objective/question: An aquatic toxicity study could be done for various reasons. It could be done to test the quality of a sediment or effluent, to register a field-applied pesticide, to understand the long-term impacts of a chemical on a community, or for routine monitoring purposes. The objective determines the type and number of tests needed.
b. Physiochemical properties of a chemical: Prior to toxicity testing it is important to collect information on the chemical’s structural formula, purity, stability in water and light, partition coefficient, vapor pressure, biodegradability, etc. because its property can influence how it moves, persists, and distributes. For example, chemicals which have poor solubility in water are more likely to accumulate in aquatic organisms or bind to sediment. However, in a lab they may instead bind to the plastic or glass container housing the test organism. Therefore, special steps need to be taken to ensure that the fate of a lab chemical mimics the fate of a chemical in the environment. Similarly, it is not worthwhile to conduct long-term toxicity studies in the lab for chemicals which degrade rapidly in the environment.
c. Mode of a chemical action: Most chemicals found in water bodies have a specific mode of action, i.e., they bind to a specific target site and produce specific downstream effects. Knowledge of a chemical’s mode of action will help decide which endpoints are the most important to measure during and after a toxicity test.
d. Pattern of chemical use (or discharge): A species can be exposed to a chemical only if there is an overlap in space and time. This means that the species must be present at a location where the chemical is found and present at a time the chemical is present in the environment. Some chemicals are only intermittently used and found in the environment. Conducting life cycle or multigenerational studies for such chemicals will not provide much usable information.
e. Environmental conditions: The environment can affect the toxicity and availability of a chemical. High temperatures can more effectively dissolve chemicals, and this might increase the amount of chemical an organism is exposed to. They can also break down chemicals, which can decrease or increase a chemical’s toxicity. Other factors can influence toxicity such as percentage of dissolved oxygen, salinity, nutrient level, moisture level, and microbial community. This makes it important to mimic the natural conditions of a test organism in the lab.
f. Characteristics of the test organism: If the objective of the study is to assess sediment toxicity, then organisms that dwell in or near the bottom of a water body should be tested rather than organisms that dwell near the top, unless a major disturbance of sediment is expected (e.g. dredging). It is necessary to choose species that can coexist together if the objective is to study how a community of aquatic species is harmed by a given chemical.
While specific guidelines for various aquatic toxicity tests were linked in Table 2, the following are general guidelines that apply to all tests:
a. Laboratory toxicity tests should be replicated at least thrice under similar conditions to ensure results are reproducible. Mesocosm tests should be replicated at least twice, while there are no requirements for macrocosm tests (due to their complexity and scale).
b. All toxicity tests should have a negative control. The control should have the same conditions and constituents as the treatment group, minus the chemical that is being tested. If the chemical is dissolved in a solvent prior to its introduction into the water, then the negative control should also contain the solvent. This is done to remove any potential effects of the solvent (though solvents should be tested before the study to ensure they are non-toxic).
c. While most toxicity tests are not required to have a positive control, it is encouraged. A positive control is a substance that is known to produce a defined toxic effect in the test organism. It is used to determine if the health and sensitivity of the test organisms have changed over the course of the study. Also, it can help validate data across different labs and help assess reproducibility of the results.
d. No less than three organisms must be treated for every concentration that is used (including control). However, the minimum sample size often depends on the study type and objective. If too few organisms are treated, it is possible to miss significant differences that might exist between treatments and controls. If too many organisms are treated (more than what is necessary), it would cause ethical issues and lead to wasted time and resources. Therefore, a power analysis is often carried out to find the minimum number of organisms needing treatment to study a particular effect.
e. All organisms used in a test must be homogenous (unless specifically instructed otherwise in the test guidelines). They should be of similar age, life stage, body mass, size, etc. They should all be healthy (sick organisms might be more sensitive to the effects of a chemical) and must have followed similar growth patterns prior to chemical exposure.
f. Randomization of controls and treatments should be done to account for non-chemical effects. For example, if all control organisms are placed on the top shelf where the light source is the brightest, they may grow differently than treatment organisms placed on the bottom shelf due to dissimilar light intensity. The difference could be mistakenly attributed to the chemical in the absence of random placement.
g. Conditions such as temperature, light, oxygen concentration, and hardness of water should be maintained throughout the test environmental to avoid impacting a chemical’s toxicity and/or availability (i.e., exposure).
h. The concentration of a chemical must be measured and maintained throughout the test. Ideally, the chemical concentration must be analyzed in the water, food, sediment, and in test organism tissues: however, most test guidelines only require that chemical concentration be measured in water.
CONCLUSIONS
[edit | edit source]While laboratory toxicity tests greatly help in understanding a chemical’s effects, the observed effects will not necessarily be the same in the natural environment. This is due to several laboratory test limitations: a. In nature, there are seasonal fluctuations, temperature changes, diverse microbial communities, etc. that are not captured in lab studies and which can greatly impact results.
b. Organisms in nature are exposed to multiple chemicals and stressors simultaneously. These chemicals and stressors can interact and produce unexpected results. The majority of lab toxicity tests are conducted with only one chemical and under conditions that are favorable to the test organism.
c. In nature, organisms may not be continually exposed to chemicals. In the lab however, long-term studies are carried out where organisms are continuously exposed to the chemical throughout their life cycle. Also, the concentration and availability of the chemical may vary in the environment while they remain constant in the lab.
d. Most lab studies involve spiking the chemical in water and exposing organisms to it. In nature, organisms are also exposed to chemicals through food and sediment.
e. Different physical habitats of ecosystems as well as the genetic structure of populations can also influence toxicity. Most lab studies are carried out on single species, ignoring species-environment, and chemical-environment interactions.
f. Lab studies are done on healthy organisms that are very similar to one another (due to frequent inbreeding). Organisms in nature are more genetically and physiologically diverse.
g. The results from surrogate species tested in the lab are extrapolated to other species since it is not possible to test the thousands of aquatic species present in nature. This adds considerable uncertainty to the results.
The first known aquatic toxicity test was conducted by Aristotle in the 4th century BC, when he exposed midge fly larvae to Athens’ effluent streams to monitor their survival and behavior. The field has progressed considerably since then, especially in the last century. Since 1899, many environmental protection laws have been introduced around the globe, and several of these laws require the regulation of chemicals prior to its registration and introduction into the environment. This has led to the development and standardization of toxicity tests. The aquatic toxicity test guidelines that are currently being used were developed within the last 27 years. However, these guidelines are not set in stone. A greater understanding of the world and man-made chemicals has led to the revision of old guidelines and the addition of new ones. In addition, separate test guidelines have been written in recent years to accommodate new chemicals with unique properties.
To better comply with increasingly stringent regulatory requirements and chemical testing while raising ethical standards, organizations such as the European Center for the Validation of Alternative Methods (ECVAM), AltTox, National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs), Center for Alternatives to Animal Testing (CAAT), etc., are focused on finding alternatives to testing chemical toxicity in animals.
A seminal report was released in 2007 by the National Academy of Sciences titled Toxicity Testing in the 21st Century: A Vision and a Strategy15. This report recommended that toxicologists move away from using vertebrates in toxicity tests and move toward the use of non-vertebrates and cell lines. Today it is possible to recreate organs on a chip and test chemicals on it16. The report also suggested using computer and mathematical models (examples include Quantitative Structure Activity Relationships, Read-Across17, Species Sensitivity Distribution18, Physiologically Based Pharmacokinetic modeling19) and knowledge-based pathways (examples include Toxicity Pathways and Adverse Outcome Pathways) to predict the toxicity of chemicals and to extrapolate results from cell lines to whole organisms and ecosystems. These technologies are yet to be implemented on a large scale, and it will probably take several decades for this to be realized.
REFERENCES
[edit | edit source]Main reference used
Rand, GM. 2008. Fish Toxicity Studies. In Di Giulio RT, Hinton DE, The Toxicology of Fishes, 1st ed. CRC Press, Taylor & Francis Group, Boca Raton, FL, United States of America, pp 659-681.
Specific in-text references
1. International Union for Conservation of Nature. Our Work-Marine. Switzerland. [cited 2019 May 5]. Available from: https://www.iucn.org/theme/species/our-work/marine
2. Reid, G. McG., Contreras MacBeath, T. and Csatadi, K. 2013. Global challenges in freshwater fish conservation related to public aquariums and the aquarium industry. International Zoo Yearbook 47(1): 6-45. Available from: https://zslpublications.onlinelibrary.wiley.com/doi/abs/10.1111/izy.12020
3. Hartung, T. 2009. Pathway Based Approaches: A European Perspective. Toxicity Pathway-Based Assessment: Preparing for Paradigm Change, a Symposium of the Standing Committee on Risk Analysis Issues and Reviews— May 11–13, 2009. National Research Council, Washington, D.C. Available from: https://www.nap.edu/read/12913/chapter/2#3
4. The American Oil Chemists’ Society. TSCA and the regulation of renewable chemicals. Urbana, IL. [cited 2019 May 5] https://www.aocs.org/stay-informed/inform-magazine/featured-articles/tsca-and-the-regulation-of-renewable-chemicals-july-august-2013
5. Nigam PK, Nigam A. 2010. Botulinum toxin. Indian J Dermatol. 55(1):8–14. doi:10.4103/0019-5154.60343. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2856357/
6. Natural Resources Defense Council. The Most Common Types of Water Contamination. New York. [cited 2019 May 5] Available from: https://www.nrdc.org/stories/water-pollution-everything-you-need-know#common
7. Ferrey M. 2013. Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes. Technical report. Minnesota Pollution Control Agency, Saint Paul, MN. Available from: https://www.pca.state.mn.us/sites/default/files/tdr-g1-16.pdf
8. Ferrey M, Streets S, Lueck A. Pharmaceuticals and Personal Care Products in Minnesota’s Rivers and Streams: 2010. Technical report. Minnesota Pollution Control Agency, Saint Paul, MN. Available from: https://www.pca.state.mn.us/sites/default/files/tdr-g1-17.pdf
9. Warner GR, Flaws JA. 2018. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicological Sciences. 166(2): 246--249. https://doi.org/10.1093/toxsci/kfy232. Available from: https://academic.oup.com/toxsci/article/166/2/246/5212891
10. National Institutes for Health, US National Library of Medicine. Cell Damage and Tissue Repair. [cited 2019 May 5] Available from: https://toxtutor.nlm.nih.gov/14-002.html
11. Browne P, Noyes PD, Casey WM, Dix DJ. 2017. Application of Adverse Outcome Pathways to U.S. EPA’s Endocrine Disruptor Screening Program. Environmental Health Perspectives. 125(9) CID: 096001. Available from: https://ehp.niehs.nih.gov/doi/10.1289/EHP1304
12. US Environmental Protection Agency, Washington, DC. Technical Overview of Ecological Risk Assessment: Risk Characterization. [cited 2019 May 5] Available from: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-risk
13. US Environmental Protection Agency, Washington, DC. Models for Pesticide Risk Assessment. [cited 2019 May 5] Available from: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/models-pesticide-risk-assessment
14. US Environmental Protection Agency, Washington, DC. Exposure Assessment Tools by Tiers and Types - Screening-Level and Refined [cited 2019 May 5] Available from: https://www.epa.gov/expobox/exposure-assessment-tools-tiers-and-types-screening-level-and-refined
15. National Research Council of the National Academies. 2007. Toxicity Testing in the 21st Century. National Academies Press. Available from: https://www.nap.edu/read/11970/chapter/1
16. Kwon D. 2017. Organs on Chips. [cited 2019 May 5] Available from: https://www.the-scientist.com/news-opinion/organs-on-chips-31020
17. AltTox.org Non-Test Approaches: (Q)SARS, READ-ACROSS [cited 2019 May 5] Available from: http://alttox.org/mapp/emerging-technologies/non-test-approaches-qsars-read-across/
18. US Environmental Protection Agency Washington, DC. Species Sensitivity Distributions. [cited 2019 May 5] Available from: https://www.epa.gov/ceam/species-sensitivity-distributions
19. US Environmental Protection Agency, Washington, DC. Physiologically-Based Pharmacokinetic (PBPK) Models [cited 2019 May 5] Available from: https://www.epa.gov/sites/production/files/2018-02/documents/pbpk_factsheet_feb2018_0.pdf
GLOSSARY
[edit | edit source]Actual Environmental Concentration (AEC): The measured concentration of a chemical in the environment.
Acute exposure: Short-term exposure to a chemical.
Acute toxicity test: Short-term toxicity tests that are usually 24-96h long.
Additive effect: Combined effect of two or more chemicals is equal to the sum of their individual effects.
Adverse Outcome Pathway (AOP): A structural representation of a sequence of biological events that leads to adverse effects, from the cellular level to the population level.
AltTox: A website dedicated to advancing non-animal methods of toxicity testing.
American Society for Testing and Materials (ASTM) International: A nonprofit international organization that develops and publishes procedures for testing chemicals and materials.
Analytical instruments: Instruments that can measure the physical and chemical properties of compounds.
Annelids: Ringed or segmented worms. Examples include earthworms, leeches and ragworms.
Antagonistic effect: Combined effect of two or more chemicals is less than the sum of their individual effects.
Benthic species: Organisms that live in the lowest level of water body. They live in, on, or near sediments. Examples include worms, crabs, clams and lobsters.
Bimodal distribution: The observation of two peaks when the data is graphically represented, unlike a normal distribution where only one peak is observed.
Bioaccumulation: Uptake and retention of chemicals through contact (integument), respiration (gills), and ingestion (mouth).
Bioconcentration: Uptake and retention of chemicals through contact (integument) and respiration (gills).
Biodegradability: Breakdown of chemicals by microorganisms, such as bacteria and fungi.
Biomass: The total mass of organisms in a given area or volume.
Cell lines: Cells that are isolated from organisms and grown in the lab under controlled conditions.
Cell perturbation: Alteration in the functioning of the cell.
Center for Alternatives to Animal Testing (CAAT): An academic center at Johns Hopkins University that is working to find new methods to replace, reduce, and refine animal testing.
Chemical purity: The degree to which a substance is undiluted or unmixed with extraneous material. Typically expressed as a percentage (%).
Chemical stability: Tendency of a chemical to resist change or decomposition due to physical, chemical, or biological factors.
Chronic exposure: Long-term exposure to a chemical.
Chronic toxicity test: Long-term toxicity tests that last for at least 10% of the life span of the tested species.
Clean Water Act (CWA): The United States Act that prohibits discharge of toxic pollutants in toxic amounts in water bodies.
Concentration of solution: The ratio of solute to solvent, or the ratio of solute to total solution.
Concentration-Response Curve: Graphical representation of a population’s response to a range of chemical concentrations.
Confidence interval 95%: A range of values within which the true value lies, with 95% certainty.
Crustaceans: Invertebrate animals with external skeleton and a segmented body. Examples include crabs, lobsters, shrimps, and prawns.
Dose: The amount of chemical that enters an organism.
Dry deposition: The direct deposition or settling of chemicals present in the air.
Early-life-stage toxicity test: Toxicity tests that are done on embryos or on larval (juvenile) stages of a species.
Ecological balance: The equilibrium between, and harmonious coexistence of, organisms and their environment.
Ecosystem: A biological community of interacting organisms and their physical environment.
Effective Concentration 50 (EC50): Concentration at which 50% of the organisms in a population are affected following chemical exposure.
Effluent: Liquid waste or sewage discharged into a water body.
Emergence: The appearance of adult insect from its pupal case (i.e., its chrysalis or cocoon).
Empirical data: Evidence gathered from observation or experimentation.
Endangered Species Act (ESA): The United States Act that requires federal agencies to ensure that their actions do not jeopardize the existence of any threatened or endangered species.
Epidermal surface: Tissue that covers the outer surface of plants.
Estimated Environmental Concentration (EEC): The estimated (non-measured) concentration of a chemical in the environment.
European Centre for the Validation of Alternative Methods (ECVAM): An European Union Reference Laboratory that is studying alternatives to animal testing.
Exposure (or exposure concentration): The concentration of chemical an organism encounters in the environment.
Extrapolate: A method to estimate new (unknown) values from old (known) values.
Filter media: Anything placed in a filter that changes the quality of water flowing through it.
Flow-through system: A setup in which organisms are held in continuously flowing water for the duration of the study.
Food web: A system of interdependent food chains. A food chain is a hierarchical series that shows how various organisms obtain food.
Geometric mean: The geometric mean of two numbers is the square root of the product (multiplication) of the two numbers.
Habitat: The natural home or environment of an animal, plant, or other organism.
Halogens: A group of reactive nonmetallic elements that include fluorine, chlorine, bromine, iodine, and astatine.
Histopathology: Microscopic examination of tissues in order to study damage.
Hydrophilic chemicals: Chemicals that easily dissolve in water and typically contain many hydrogen and oxygen atoms.
Hydrophobic chemicals: Chemicals that do not dissolve in water and typically contain many carbon atoms.
Inhibitory Concentration 50 (IC50): Concentration at which 50% of the organisms in a population are inhibited following chemical exposure.
Influent: Something that flows into a system.
International Organization for Standardization (ISO): An independent international organization that sets standards for many materials, products, processes, and services.
Kelp: A type of large brown seaweed.
KOW (Based) Aquatic Bioaccumulation Model (KABAM): A model that can estimate potential bioaccumulation of hydrophobic carbon-based pesticides in freshwater aquatic food webs.
Lethal Concentration 50 (LC50): Concentration at which 50% of the organisms in a population are killed following chemical exposure.
Life cycle toxicity test: Toxicity tests that last throughout the life cycle of an organism.
Lowest Observed Effect Concentration (LOEC): The lowest concentration of chemical that causes a toxic effect in the treated population.
Macrocosm: Toxicity tests that are conducted in lakes and on whole aquatic ecosystems.
Maximum Acceptable Toxicant Concentration (MATC): The concentration between NOEC and LOEC, or a geometric mean of the two.
Mesocosm: Toxicity tests that are conducted on multiple species placed in experimental water enclosures that mimic natural conditions. The system contains more than 1000 liters of water.
Metabolic byproducts (or metabolic waste): The left-over products of metabolism.
Metabolic enzymes: Enzymes that carry out a variety of critical cellular functions in organisms.
Metabolism: The chemical processes that occur within a living organism in order to maintain life.
Metamorphosis: The process of transformation of an organism from an immature form to an adult form.
Microcosm: Toxicity tests that are conducted on two or more species in an artificial and controlled system within a laboratory or outdoors. The system contains less than 1000 liters of water.
Model organism: A species that has been widely studied, is easy to maintain and breed in a laboratory, and has several other characteristics that make it ideal for experimental use.
Mode of action: The mechanism by which a substance produces an effect in living organisms or biochemical systems.
Moles: The quantity of a substance that has the same number of particles found in 12 grams of carbon-12 (6.02x1023 particles).
Mollusks: Invertebrate animals that have a soft unsegmented body. Examples include snails, slugs, mussels, and octopuses.
Multigenerational toxicity tests: Toxicity tests that are carried out on two or more consecutive generations (parents and offspring).
National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs): A UK-based scientific organization dedicated to finding alternatives to animals in research and testing.
Negative control: A group that has the same conditions and constituents as the treatment group, minus the chemical that is being tested.
No Observed Effect Concentration (NOEC): The highest chemical concentration that does not cause a toxic effect in the treated population.
Normal distribution: The distribution of many random values as a symmetrical bell-shaped graph.
Organization for Economic Co-operation and Development: An intergovernmental economic organization that considers the environmental implications of economic and social development.
Partition coefficient: The ratio of concentrations of a chemical in a mixture of two immiscible (unmixable) liquid phases at equilibrium.
Parts per billion: Corresponds to 1 µg of chemical/L of solution. The amount of chemical is nine orders of magnitude lesser than the amount of solution.
Parts per million: Corresponds to 1 mg of chemical/L of solution. The amount of chemical is six orders of magnitude lesser than the amount of solution.
Parts per trillion: Corresponds to 1 ng of chemical/L of solution. The amount of chemical is twelve orders of magnitude lesser than the amount of solution.
Peak concentration in water: The maximum concentration of chemical estimated or measured in water.
Pesticide: A substance used to destroy pests. The pests can be insects, weeds, fungi, rodents, etc.
Pesticide in Water Calculator (PWC): A model that estimates pesticide concentrations in water bodies following pesticide applications to land.
Physiochemical properties: The properties of a chemical observed from its interaction with the physical environment.
Physiologically based pharmacokinetic (PBPK) modeling: A mathematical modelling technique that predicts the absorption, distribution, metabolism, and excretion of substances in living organisms.
Plankton: A diverse collection of small organisms that live in large bodies of water and are unable to swim against a current. Examples include bacteria, algae, protozoa, and archaea.
Positive control: A group that receives a substance that is known to produce a defined toxic effect.
Potentiation effect: Combined effect of two or more chemicals is greater than the sum of their individual effects. Usually one of the chemicals produces no effect on its own.
Power analysis: A statistical analysis that helps determine the sample size required to detect an effect of a given size with a given degree of confidence.
Quantitative structure–activity relationship (QSAR) model: A model that predicts the biological activities of untested chemicals using their structures.
Randomization: The process of making something random to minimize bias.
Read-Across: A model that predicts the toxicity of untested chemicals using the toxicity information of similar but tested chemicals.
Recirculation system: A setup in which organisms are held in water that is continuously run through a filter for the duration of the study.
Renewal system: A setup in which organisms are held in still water that is regularly changed for the duration of the study.
Resistance: The natural ability of some organisms to withstand the effects of a compound.
Risk: It is the likelihood of a hazard (e.g. a toxic chemical) causing harm.
Risk Quotient: The ratio of chemical exposure to chemical effects.
Sample size: The number of organisms in an experimental sample or group.
Sediment toxicity testing: Toxicity tests where aquatic organisms are exposed to sediments to determine if the chemicals in the sediments will harm them.
Sensitivity: An organism’s susceptibility to the effects of a compound.
Sigmoidal curve: A “S” shaped curve.
Site of action: The location where a compound binds to exert its effects. This is usually a cell receptor, an ion channel or an enzyme.
Slope of curve: The steepness of a curve.
Solvent: A liquid in which a compound is dissolved to form a solution.
Species diversity: The number and distribution of species in the environment.
Species richness: The number of species in the environment.
Species Sensitivity Distribution: A mathematical model that describes the variation in chemical sensitivity between species.
Spiking: The addition of a compound to a solution.
Standard deviation: A quantity that indicates how measurements for a group are spread out from the average or expected value.
Standardization: The process of making something conform to a standard i.e. done according to specific guidelines.
Static system: A setup in which organisms are held in static water that in unchanged for the duration of the study.
Stomata: Minute pores in the epidermis of the leaf or stem of a plant which allows for exchange of gases.
Stressors: Factors that stress organisms. Examples include food shortage, parasites, predators, and changing environmental conditions.
Subchronic toxicity test: Prolonged acute toxicity tests that are typically between 28 days to 3 months long in fish.
Surrogate species: A species that is used to represent multiple species or aspects of the environment.
Swimming equilibrium: The ability that allows fish to maintain an upright position within the water column.
Synergistic effect: Combined effect of two or more chemicals is greater than the sum of their individual effects.
Threshold concentration: The lowest concentration of a chemical that elicits a response.
Tiered Testing: A toxicity testing method where lower tiered studies (representing simple worse-case scenarios) are followed by higher tiered studies (representing complex realistic scenarios).
Toxic effects: The adverse effects produced by exposure to a substance.
Toxicity: The degree to which a substance can harm living organisms.
Toxicity curve: A graph in which chemical concentration (or dose) is plotted against an organism response.
Toxicity endpoint: The measurements and observations taken during or after a toxicity study.
Toxicity Pathway: A sequence of intracellular events that, if disturbed, can cause an adverse outcome at the cellular level.
Toxicity test: A test conducted to find the degree to which a substance can harm living organisms.
Toxicity thresholds: The lowest concentration of a chemical that causes toxicity in organisms.
Uncertainty: Undetermined factors that can produce unreliable results.
United States Environmental Protection Agency (USEPA): A federal agency in the United States that is responsible for registering and regulating chemicals, including pesticides.
Validate: Check or prove the accuracy of (something).
Vapor pressure: A quantity which informs the tendency of a chemical to evaporate.
Vascular system: The presence of xylem and phloem tissues in plants that are responsible for transporting water, nutrients, and food.
Wet deposition: The deposition or settling of chemicals in the air through rainfall, snowfall, and fog.
Whole effluent toxicity testing: Toxicity tests where aquatic organisms are exposed to wastewater effluents to determine if chemicals in the effluents will harm them.
